Clinical assessment of ALPHA BRIGHT SERUM vs HYDROQUINONE 2%
Clinical assessment of ALPHA BRIGHT SERUM vs HYDROQUINONE 2%
«6 MONTHS CLINICAL TRIAL TO ASSESS THE DEPIGMENTING EFFICIENCY OF A STABLE ANTIOXIDANT SERUM VERSUS HYDROQUINONE 2% WITH 40 PATIENTS ON FACIAL HYPERPIGMENTATION»
Dra Natalia Guadalupe Pérez Leal*
Dra María Ivonne Arellano Mendoza*
Dra. Rosa María Ponce Olivera*
Dra. Olga Labastida Gomez De la Torre**
*Hospital General de México Dr. Eduardo Liceaga, Mexico
** Clínica Sensalaser, Hospital Angeles Pedregal, Mexico
_____________________________________________________________________________________________
This study is an evaluation of the depigmenting efficacy of an antioxidant serum (ALPHA BRIGHT SERUM®) versus a reference treatment (Hydroquinone 2%) in 40 patients with melasma and post-inflammatory pigmentation during 6 months.
This clinical trial reveals both treatments used have similar efficacy. An average severity decrease of 28% was observed in melasma and 41% severity decrease in post-inflammatory hyper-pigmentation. The best responses were observed in melasma patients, where some patients presented improvements of 71%.
_____________________________________________________________________________________________
BACKGROUND
Melanogenesis accomplished by melanocytes is a complex process in which tyrosinase converts precursor L-tyrosine to L-DOPA by hydroxylation, so that it becomes L-DOPA quinone by oxidation. Furtherly it is transformed in eumelanin or pheomelanin, then pigment is transferred to keratinocytes by phagocytosis [1]. We will focus on 2 main hyperpigmentation disorders: melasma and post-inflammatory hyperpigmentation (PIH). MELASMA Melasma is an acquired, chronic, recurrent and symmetrical hyper melanosis, predominantly located on the facial area and characterized by sharply demarcated brown and greyish maculae. This pigmentary disorder is more common in women than in men, it appears in all skin phototypes, especially in phototypes III to IV, it is more frequent in persons living in areas subjected to intense ultraviolet radiation. Its prevalence varies between 1.5 and 33.3% depending on the population [2]. The causes of melasma are not fully understood, but triggers can include genetic, hormonal, and UV exposure. The predilection for women suggests the involvement of hormonal factors, pregnancies and the use of oral contraceptives. During pregnancy, the levels of hormones that stimulate melanogenesis increase. By inducing the synthesis of melanogenic enzymes such as tyrosinase and tyrosinase- related proteins 1 and 2, oestrogen stimulates melanogenesis in human melanocytes in cultures [3]. Recent findings suggest that thyroid hormones may play a key role in melasma. This idea has also been supported by some epidemiological studies other factors involve vascular dilation, basal membrane alteration and exposure to visible light. Kimbrough-Green proposed to focus on the Melasma Area and Severity Index (MASI) to clinically quantify the severity of facial melasma.
POST-INFLAMMATORY HYPERPIGMENTATION
Post-inflammatory hyperpigmentation (PIH) is an acquired and common pigmentary disorder caused by cutaneous inflammation secondary to underlying dermatosis or cutaneous procedures. Inflammatory dermatoses can cause clinical hyperpigmentation, hypopigmentation, or both. PIH can present after different etiologies, such as psoriasis, lichen planus, infectious dermatoses, drug reactions, surgical procedures, burns and trauma [4]. One of the most frequently cause observed in our population is post acne PIH, and we use the visual evaluation through the Post Acne HyperPigmentation Index (PAHPI) that is easily performed, considering the normal skin colour at baseline. Wood ́s lamp illumination is a simple and useful diagnostic tool in evaluation of the extension of pigmented areas.
TREATMENTS
Several agents have been proposed to impact in different stages of melanogenesis and some of their mode of action include: inhibition of melanin production and melanosome transfer, increased turnover of keratinocytes, anti-inflammatory and antioxidant effects. Different compounds or combination therapies for melasma have been proven effective. Hydroquinone (HQ) is a first-line treatment for melasma. It is the most widely used depigmenting agent [5]. Oral tranexamic acid (TXA) has proven to be a promising treatment for recalcitrant melasma. It is the first systemic therapy for melasma, it was first described as a potential therapy in 1979 and demonstrated improvement after 4 weeks of treatment [5]. Other depigmenting treatments include topical retinoids, azelaic acid, arbutin, niacinamide, ascorbic acid and N-acetylglucosamine; none of these treatments will achieve satisfactory depigmenting results unless appropriate and effective photoprotection measures are prescribed an observed by patients, and the use of broad spectrum sunscreens, effective against visible light are a mainstay in treatment. Due to the therapeutic challenge associated with this type of pathology and sometimes the poor response to the treatments mentioned, new depigmenting combinations have been proposed, including the antioxidant serum comprising the standardized combination of: 2% Phytic acid, 8% stabilized ascorbic acid, 1% acetyl Glycyl beta alanine, 30% Ginkgo Biloba solution (ALPHA BRIGHT®).
Phytic acid is an organic acid extracted from rice grain that has antioxidant, moisturizing, depigmenting and sebum regulating properties [6]. When stabilized, Vitamin C is one of the most potent topical antioxidants known and due to its properties it has been shown to protect against collagen degradation and also exhibits lightening properties of the skin. Its clinical applications range from photoprotection to the anti-aging and anti-pigmentation strategy [7]. Acetyl Glycyl beta alanine is a peptide that exhibits a fast skin penetration, with excellent results in reducing melanin production and inhibiting its transference to keratynocytes. Among its mechanisms of action describes it figures the inhibition of stem cell factor and endothelin 1 which reduces the enzymes generating melanin (tyrosinase, proteins related to tyrosinases 1 and 2). It has been found to decrease the transcription factor associated with microfalalmie (MITF) [8].
OBJECTIVE OF THE STUDY
The primary objective was to evaluate the effectiveness to improve severity in melasma and post-inflammatory hyperpigmentation of two topical depigmenting products: ALPHA BRIGHT SERUM® (ALPHASCIENCE) (Acetyl-Glycyl-B-Alanine + 8% ascorbic acid + 2% acid Phytic + Ginkgo Biloba) versus a cream containing 2% Hydroquinone. The secondary objectives was to evaluate the satisfaction and clinical age calculated by Skin vision VisiaBooth of the patients with the use of both products.
METHODOLOGY
A prospective, randomized, open label trial was designed and the Research and Ethics Committee approved the protocol. They were recruited 20 melasma patients and 20 patients with facial PIH. As it was designed as a split face study, the patients were their controls. The study was designed in a three-phase modality for 6 months duration. For the first eight week patients were randomly assigned to application of the topical agent on half of the face, so ALPHA BRIGHT SERUM® (AB) was applied by gentle massage on the evenings and in the other half of the face the patient applied HYDROQUINONE 2% cream (HQ). After 8 weeks the product was exchanged to the other half of the face. Finally all patients applied ALPHA BRIGHT SERUM® (AB) at morning and night for additional 8 weeks. In addition, all of the patients applied the same SPF 50 broad-spectrum sunscreen at morning and midday. Population and sample size: 20 melasma patients and 20 patients with post- inflammatory pigmentation, were included in our study, the age range was 25 to 60 years.
All of the patients were evaluated through Wood’s light and quantitative evaluation by Skin vision Visia Booth, so colorimetry, number and depth of wrinkles, pore size and texture of the skin as well as the calculated clinical age values were compared. MASI was calculated for melasma patients and PAHPI for PIH patients at each visit, as well as digital iconography. All of the patients included, answered a questionnaire for evaluation of the products applied and satisfaction. Basal (T0), 8 weeks (T2), 16 weeks (T3) and 24 weeks (T4) results were analysed.
Statistical Analysis: The general data were analysed using descriptive statistics, mean and standard deviation for the quantitative variables with normal distribution, percentiles in the case of those that are not and frequencies and percentages for the categorical variables. Effect size measured by the d (Cohen) index. It was used the statistical program SPSS V.24 for Mac (IBM, Chicago, II), and it was considered that there was a statistically significant difference when the value of p was < 0.05.
RESULTS
-
MELASMA
The overall MASI measurement of the whole face showed an average decrease of 28% after 6 months (p 0.204) with a MASI score decreasing from 19 to 14. The comparison between the 2 treatments HYDROQUINONE 2% and ALPHA BRIGHT SERUM® showed equal reduction of pigmentation after 2 months (-11% VS -12%) and 4 months (-14% VS -12%). The decrease continued between 4 months and 6 months by -14% when exclusively treated with ALPHA BRIGHT SERUM®. According to the graph below we can note the decrease of the MASI score by HYDROQUINONE 2% treatment and ALPHA BRIGHT SERUM® at T0, T2 months, T4 months and T6 months. The evolution of the calculated age also shows a decrease. We can note a rejuvenation of 4 months between the T0 and T6 months of treatment.
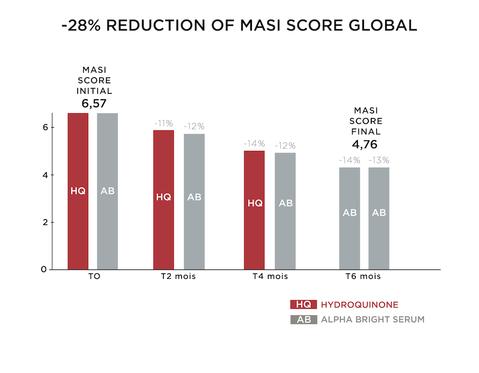
-
POST INFLAMMATORY HYPERPIGMENTATION
The measurement of PAHPI on both sides of the face showed a decrease of 41% (p 0.0001) after 6 months, decreasing from 13.2 to 7.7. According to the graph below we can note the decrease of the PAHPI score by HYDROQUINONE 2% treatment and ALPHA BRIGHT SERUM® at T0, T2 months, T4 months and T6 months. The evolution of the calculated age also showed a decrease. We can note a rejuvenation of 4 months after 6 months of treatment.
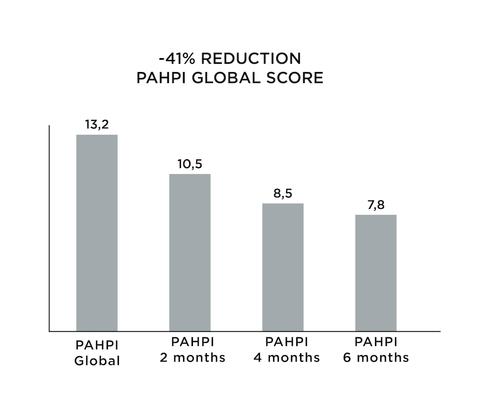
-
CONCLUSIONS
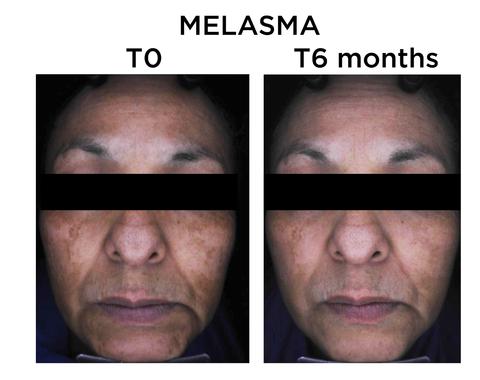
Hyperpigmentation T0 T6 months Case n°11 – Post inflammatory hyperpigmentation Both treatments used for 6 months have similar efficacy on post-inflammatory hyperpigmentation and melasma. An average decrease of 28% was observed in melasma and 41% decrease in post-inflammatory pigmentation. One patient achieved a decrease in pigmentation up to 71% on melasma.
The ALPHA BRIGHT SERUM® has antioxidant depigmenting action, also decreased pigmentation after the use of hydroquinone 2% treatment. It can therefore be an interesting option after hydroquinone treatment to manage residual pigmentation and also as a maintenance therapy for melasma and PIH. Skin texture improved and patients referred satisfaction with the treatment with this serum.
_____________________________________________________________________________________________
REFERENCES
(1) Zhou LL, Baibergenova A. Melasma: systematic review of the systemic treatments. International Journal of Dermatology. 2017:1-7.
(2) Arellano MI, Ocampo J, Rodríguez M et al. Guias de diagnóstico y manejo de melasma. Dermatología CMQ. 2017;16(1):12-23.
(3) Filoni A, Mariano M, Cameli N. Melasma: How hormones can modulate skin pigmentation. J Cosmet Dematol. 2019;1-6.
(4) González N, Robles J, Ocampo J. Artículo de revisión: hiperpigmentaciones adquiridas. Dermatología CMQ. 2017;16(1):50-62.
(5) Zubair R, Lyons A, Vellaichamy G et al. Whats new in pigmentary disorders? Dermatol Clin.2018:1-8.
(6) Zhou JR, Erdman JW. Phytic Acid in Health and Disease. Critical Reviews in Food Science and Nutrition. 1995; 35: 495-508.
(7) Kishimoto Y, Saito N, Kurita, K et al. Ascorbic Acid Enhances the Expression of Type 1 and Type 4 Collagen and SVCT2 in Cultured Human Skin Fibroblasts. Biochemical and Biophysical Research Communications. 2013; 430: 579-584
(8) Glycine derivative capable of inhibiting melanin formation and composition using the same (US 8,927,499 B2) https://www.google.com/patents/US8927499 Crystalline polymorphs of acetyl-glycine-beta-alanine and process of making the same (US 8,927,767 B2) https:// www.google.ch/patents/US20130195782?hl=de

